RAPID trial is a multinational, open-label, phase 4, individually-randomised trial. Patients will be randomised to either a control or intervention arm. Patients randomised to the intervention arm will have relevant specimens analysed by rapid diagnostics with early availability of ceftazidime-avibactam, if appropriate.
This trial has been registered on clinicaltrials.gov:
https://clinicaltrials.gov/study/NCT05979545
Study Design & Objectives
The RAPID trial proposes a seamless intervention linking rapid bacterial isolate identification and antibiotic resistance gene detection and prompt administration of targeted antibiotic to minimise time between infection onset and appropriate treatment.
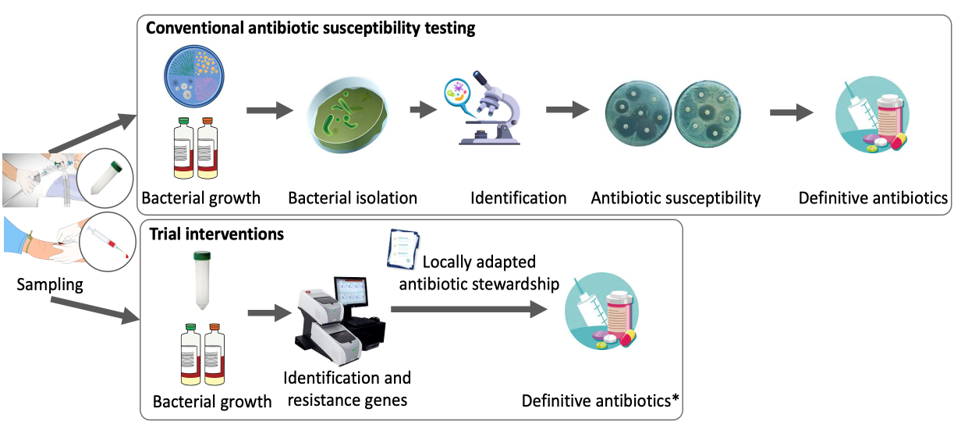
The primary hypothesis is that these interventions will lead to improved clinical outcomes amongst patients with hospital-acquired bloodstream infection, hospital-acquired pneumonia or ventilator-associated pneumonia due to carbapenem non-susceptible Pseudomonas aeruginosa or Enterobacterales, compared to standard antibiotic susceptibility testing.
Enrolment, Sample Size and Sites
The main population that will be recruited in the study will be hospitalised patients with bloodstream infections, hospital-acquired pneumonia or ventilator-associated pneumonia due to Pseudomonas aeruginosa or carbapenemase producing Enterobacterales treated with ceftazidime-avibacatam, while the secondary population recruited will be those with multidrug resistant (MDR) Gram-negative bacilli. The total target sample size is 1900 participants in the main population over 20 study sites.
Informed Consent Video

Copyright © ADVANCE-ID. All rights reserved.
ADVANCE-ID
Saw Swee Hock School of Public Health
National University of Singapore
Tahir Foundation Building
National University of Singapore
12 Science Drive 2, #10-01, Singapore 117549
+65 6516 4988